Categorised under:
Anaesthesia
>
Patient monitoring
>
Cardiac output monitors

Did you know you can Register for FREE with this website?
Registration gives you full access to all of the features of WhichMedicalDevice. Find out more ...
WhichMedicalDevice is a FREE resource created by clinicians for clinicians.
Registration is free and gives you unlimited access to all of the content and features of this website.
Find out more...Registration is free and gives you unlimited access to all of the content and features of Which Medical Device. Find out more...
Which Medical Device is a community of clinicians sharing knowledge and experience of the devices and procedures we use on a daily basis. We ask that our members register with us so that we can maintain the unbiased and independent nature of our content. Registration is quick and free.
We do not make your details available to any third parties nor do we send unsolicited emails to our members. You can read our Privacy Policy here.
Comments
Comment by brendansmith Commented Jan 9, 2014
Impact factor: 54
User Rating
Not bad in our tests, but not close enough for a prize. Not that suitable for everybody as swallowing the probe is not all that easy, especially in kids and confused and uncooperative patients. Much used in anaesthesia and ICU where these considerations are not so important. Accuracy probably about 80%, which is better than most things out there...
Professor Brendan Smith, Australia.
Comment by Jeremy_Hyams Commented Jul 25, 2013
Impact factor: 112
User Rating
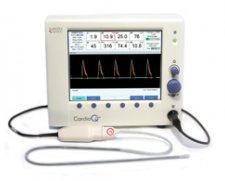
Interest has been growing over the last decade in non-invasive cardiac output monitoring (NICOM) devices, particularly the CardioQ after being endorsed by NICE. The most recent guidance from NICE in March 2011 states “The CardioQ-ODM should be considered for use in patients undergoing major or high-risk surgery… in whom a clinician would consider using invasive cardiovascular monitoring.”
I’ve been using it (as well as other NICOM devices for a number of years), and have found it fairly easy to use & configure. Turn the machine on and plug in a new probe, it will automatically ask for patient demographics before taking you straight to the cardiac output measurement screen. Vital parameters are presented in a straightforward manner on the same screen without needing to navigate through various layers / equations to get hold of the data you need.
A downside of this device is that it relies entirely on placing an oesophageal doppler probe at a fairly exact point within the oesophagus to be able to measure blood flow within the aorta. Fail to get the probe in exactly the right spot and you can’t be sure that the data you’re getting relates to the patient’s actual cardiac output. Equally, very slight changes in the position of the probe will affect the numbers on the screen, and unless you’re careful, you can act on changes due to probe position rather than the patient’s haemodynamic status. It can require a fair bit of fiddling to get a good trace on the screen, and this can distract from the rest of the anaesthetic. This isn’t an issue when using devices such as the LiDCORapid.
NICE quote cost savings per patient of £1,100 for a 7.5 day stay after major surgery, which dwarfs the cost of the probe (approximately £65). Whether the research these numbers are based on is transferrable to the real world is hard to tell, particularly as some of the evidence was provided by the device manufacturer, Deltex. These calculations also assume that a central venous catheter would not be inserted for monitoring, whereas most of the literature showing benefit with these devices looks at patients who are monitored with both NICOM & central venous pressure monitoring.
Given the NICE recommendations and the evidence for reductions in morbidity, mortality & length of stay, it becomes very hard to ignore this device. However, with regards to the cost savings, this is something individual units would have to audit over time.