Categorised under:
Interventional Radiology
>
Vascular
>
Renal denervation
The St Jude Medical EnligHTN renal denervation catheter is a monopolar multipoint electrode radiofrequency ablation catheter. There are 4 radiopaque electrodes that are arranged in an expandable basket like configuration with 2 electrodes on opposite sides of the distal basket and two further electrodes more proximally in the same arrangement. The tip of the device features a smooth nitinol cone that allows smooth passage of the catheter into the renal artery.
 The tip of the catheter can be deflected by an extendable mechanism on the device handle and the basket is expanded via a turnable dial on the device handle. The catheter is compatible with an 8Fr guiding catheter and comes in two sizes. The smaller sized catheter is suitable for 4-6mm and the larger 5.5-8mm renal arteries.
The tip of the catheter can be deflected by an extendable mechanism on the device handle and the basket is expanded via a turnable dial on the device handle. The catheter is compatible with an 8Fr guiding catheter and comes in two sizes. The smaller sized catheter is suitable for 4-6mm and the larger 5.5-8mm renal arteries.
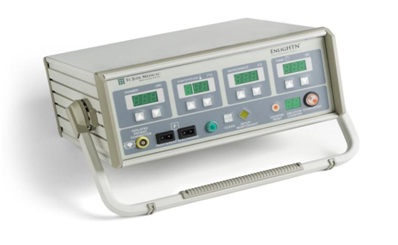 The generator displays readings for each individual electrode displaying the temperature and impedence. There is a counting timer for each ablation point and the next electrode automatically turns on once the ablation for the previous electrode finishes. The generator is not as modern in appearance and design as other CE marked systems and requires some training/experience to operate properly and to interpret the temperature and impedence readings. The generator is also quite large in size compared to other CE marked devices.
The generator displays readings for each individual electrode displaying the temperature and impedence. There is a counting timer for each ablation point and the next electrode automatically turns on once the ablation for the previous electrode finishes. The generator is not as modern in appearance and design as other CE marked systems and requires some training/experience to operate properly and to interpret the temperature and impedence readings. The generator is also quite large in size compared to other CE marked devices.
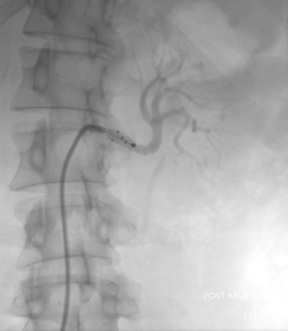
Once the catheter is positioned within the renal artery and the basket expanded injection of contrast is performed to confirm positioning in the renal artery (below). A test run of the generator should demonstrate a small increase in temperature as well as a stable impedence reading to indicate good wall contact. If there is poor wall contact the basket can be collapsed and the catheter slightly repositioned before expanding and repeating the test run. Individual electrodes can be turned off if required.
After the first set of ablations have been performed, the catheter is collapsed and then retracted 1cm before being turned approximately 90 degrees and then re-expanded to ensure different locations for the 8 ablation points. Each ablation lasts 90 seconds per electrode and the company recommends 2 sets of 4 ablations per renal artery, totalling 16 ablation points with a total ablation time of 24 minutes. This is on the longer side compared with the other currently available CE marked devices and generally requires more sedation and analgesia, if no general anaesthetic administered,due to the longer procedure time.

Did you know you can Register for FREE with this website?
Registration gives you full access to all of the features of WhichMedicalDevice. Find out more ...
WhichMedicalDevice is a FREE resource created by clinicians for clinicians.
Registration is free and gives you unlimited access to all of the content and features of this website.
Find out more...Registration is free and gives you unlimited access to all of the content and features of Which Medical Device. Find out more...
Which Medical Device is a community of clinicians sharing knowledge and experience of the devices and procedures we use on a daily basis. We ask that our members register with us so that we can maintain the unbiased and independent nature of our content. Registration is quick and free.
We do not make your details available to any third parties nor do we send unsolicited emails to our members. You can read our Privacy Policy here.