Published date : 28 June 2012
Article date : 28 June 2012
Editorial by Dr Ondina Bernstein, Specialist Registrar Radiology, and Dr Nicholas Chalmers, Consultant Vascular Radiologist, both of Central Manchester University Hospitals NHS Foundation Trust
Recent developments in techniques and endovascular devices have led to an improvement in the outcomes of endovascular treatment of infrapopliteal disease. The main indication for treatment is critical limb ischaemia (CLI) rather than claudication. Limb salvage therefore tends to be the rationale for treatment. The pathogenesis differs from larger vessel disease, with a higher prevalence of diabetes and renal failure. Venous disease and neuropathy may contribute to the aetiology of ulceration, necessitating a multi-factorial management approach. Because patients have many co-morbidities, endovascular treatment is often considered as the first option in the treatment of CLI, but should be considered an adjunct to drug therapy. The smaller calibre vessels and longer segments of involvement seen in infrapopliteal disease require different tools to those used in larger vessels. High technical success rates are achieved with modern low profile and longer segment angioplasty balloons, which are used over 0.014 or 0.018 wires. However, recent randomised controlled trials (RCTs) suggest that drug-eluting stents (DESs) achieve improved patency over plain balloon angioplasty or bare metal stents. This short review summarises some of the devices that are available in the treatment of infrapopliteal disease management.
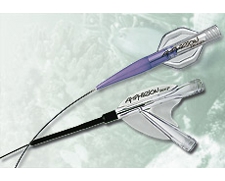
Plain-balloon Angioplasty
Straight-line pulsatile flow to the foot is considered to be the goal of infrapopliteal intervention. However, the greater the number of patent vessels, the greater the chance of limb salvage. Infrapopliteal angioplasty is associated with high technical success rates when using smaller diameter wires and balloon technologies (1,2). Limb salvage rates are equivalent to bypass surgery, although the durability of angioplasty is significantly lower (3). The majority of amputations are performed in the first year after intervention and thereafter the rate is less than 1% per year (4). Longer segment and smaller diameter
balloons (1.5 – 4mm diameter), suitable for infrapopliteal intervention, are available from many companies, e.g.
Amphirion Deep (Medtronic Invatec, Frauenfeld, Switzerland);
Advance (Cook Medical, IN, USA);
Fox sv (Abbott Vascular, Illinois, USA). The “KrossLock” inner metal shaft of the
ReeKross balloon (ClearStream Technologies Ltd, Wexford, Ireland) results in improved pushability and torque, useful in infrapopliteal intervention.
Drug-eluting balloons (DEBs)
Paclitaxel is used as an active agent in drug-eluting balloons, is realeased into the media at the site of angioplasty and reduces restenosis due to neo-intimal hyperplasia by suppressing the proliferation of smooth muscle cells. Interim results from the DEBATE-BTK study using the
In.Pact Admiral paclitaxel-eluting balloon (Medtronic Invatec, Frauenfeld, Switzerland. Not FDA approved) demonstrate significantly lower restenosis (27% vs. 65%) and reocclusion (16% vs. 53%) rates compared with plain PTA (5). At least two further randomised trials of DEB vs. plain balloon angioplasty are currently recruiting (
IN.PACT DEEP and
EURO-CANAL).
 Atherectomy Devices
Atherectomy DevicesAtherectomy devices physically remove the obstructing lesion. The
SilverHawk directional atherectomy device (EV3, Minneapolis, MN) contains a high-speed carbide-cutting disc that cuts long ribbons of atheroma, which are then stored in the nose-cone of the device. Twelve-month primary and secondary patency rates of up to 67% and 91% have been published (6). Pre-dilatation and adjunct PTA/stenting were required for a number of the lesions.
Laser-assisted angioplasty (laser atherectomy) using the CLiRpath 308-nm wavelength excimer system (Spectranetics, Colorado Springs, Colo) achieved limb salvage rates of 93% at 6 months (7,8).
The
Diamondback 360 Orbital Atherectomy System (Cardiovascular Systems Inc, St Paul, MN) uses a plaque ablation catheter. This has an abrasive eccentrically shaped crown with a diamond coated surface which rotates and creates lumen enlargement by plaque abrasion. A multi-centre registry foundthat 90% achieved <30% final diameter stenosis and no major amputations at six-months (9).
 Cryoplasty balloons
Cryoplasty balloonsCryoplasty uses the controlled induction of apoptosis in the vessel wall by the cold balloon, with the aim that smooth muscle cell proliferation and restenosis is reduced. The non-randomised Chill trial utilised the
PolarCath cryoplasty system (Boston Scientific, Natick, MA, USA). Major amputation was avoided in 85% of patients at one-year (10).
Bare metal stents (BMSs)
The majority of published studies involve the use of balloon-expandable coronary stents in the calf vessels [eg. 11]. Stents specifically designed for the calf vessels include small
diameter self-expanding stents, e.g
Maris Deep (Invatec-Medtronic Frauenfeld, Switzerland),
X-pert (Abbott Vascular, Illinois, USA) and longer-length balloon expandable stents (e.g.
Chromis Deep, Invatec-Medtronic Frauenfeld, Switzerland, not FDA approved). There is no level 1 evidence to support primary bare-metal stenting over angioplasty. BMS placement has therefore been recommended to be reserved for suboptimal results after angioplasty such as residual stenosis or flow limiting dissection.
Bio-absorbable Stents
Bio-absorbable stents aim to mechanically prevent vessel recoil without the permanent presence of an artificial implant that could be a potential trigger for restenosis. The magnesium-alloy bio-absorbable stent (Biotronik, AG) did not show any improvement in long-term patency over PTA (12).
 Drug-eluting stents (DESs)
Drug-eluting stents (DESs)A local high dose of the anti-proliferative drugs can be achieved without systemic toxicity. The common drugs are sirolimus, everolimus and paclitaxel. These work at different points in the cell division cycle and are intended to suppress the smooth muscle cell proliferation that occurs as a consequence of trauma to the vessel wall. In recent RCTs, DESs have achieved significantly improved primary patency rates over either BMSs or angioplasty alone, although improved limb salvage and ulcer healing rates have not been convincingly demonstrated. Table 1 summarises the RCTs of DESs.
Table. 1
RCT
| Procedure
| Drug-eluting Stent
| Primary outcome measures
| Primary outcome conclusion
|
(13) | Abciximab plus Sirolimus eluting stent /BMS/PTA/PTA alone
| Cypher select (Cordis Corporation, Bridgewater, New Jersey)
| Feasibility trial
| Reduced restenosis in DES group
|
(14) | Sirolimus-eluting stents vs. BMS
| Yukon (Translumina, Hechingen, Germany). Not FDA approved.
| Primary patency rate (freedom from in-stent restenosis) (>50%) at 12-months
| Significantly higher primary patency in DES group
|
DESTINY 2012
(15) | Everolimus-eluting stent vs. BMS
| Xience V (Abbott Vascular, Illinois, USA)
| Primary patency rate (freedom from binary in-stent restenosis) (>50%) at 12-months
| Significantly lower restenosis rate in DES group
|
(16) | Sirolimus-eluting stent vs. PTA | Cypher select (Cordis Corporation, Bridgewater, New Jersey) | In-stent binary restenosis at 12-months
| Significantly lower restenosis rate in DES group |
Summary
Recent advancements in endovascular devices available for the treatment of infrapopliteal disease, have led to improved technical success rates. A consensus on the optimal treatment approach has not been reached. DESs appear to be achieving the best outcomes in recent RCTs. However, improved outcomes in terms of limb salvage and ulcer healing, which tends to be the goal of infrapopliteal intervention has not yet been convincingly demonstrated.
User comments: Infrapopliteal disease management
References

 balloons (1.5 – 4mm diameter), suitable for infrapopliteal intervention, are available from many companies, e.g. Amphirion Deep (Medtronic Invatec, Frauenfeld, Switzerland); Advance (Cook Medical, IN, USA); Fox sv (Abbott Vascular, Illinois, USA). The “KrossLock” inner metal shaft of the ReeKross balloon (ClearStream Technologies Ltd, Wexford, Ireland) results in improved pushability and torque, useful in infrapopliteal intervention.
balloons (1.5 – 4mm diameter), suitable for infrapopliteal intervention, are available from many companies, e.g. Amphirion Deep (Medtronic Invatec, Frauenfeld, Switzerland); Advance (Cook Medical, IN, USA); Fox sv (Abbott Vascular, Illinois, USA). The “KrossLock” inner metal shaft of the ReeKross balloon (ClearStream Technologies Ltd, Wexford, Ireland) results in improved pushability and torque, useful in infrapopliteal intervention.  Atherectomy Devices
Atherectomy Devices Laser-assisted angioplasty (laser atherectomy) using the CLiRpath 308-nm wavelength excimer system (Spectranetics, Colorado Springs, Colo) achieved limb salvage rates of 93% at 6 months (7,8).
Laser-assisted angioplasty (laser atherectomy) using the CLiRpath 308-nm wavelength excimer system (Spectranetics, Colorado Springs, Colo) achieved limb salvage rates of 93% at 6 months (7,8). Cryoplasty balloons
Cryoplasty balloons diameter self-expanding stents, e.g Maris Deep (Invatec-Medtronic Frauenfeld, Switzerland), X-pert (Abbott Vascular, Illinois, USA) and longer-length balloon expandable stents (e.g. Chromis Deep, Invatec-Medtronic Frauenfeld, Switzerland, not FDA approved). There is no level 1 evidence to support primary bare-metal stenting over angioplasty. BMS placement has therefore been recommended to be reserved for suboptimal results after angioplasty such as residual stenosis or flow limiting dissection.
diameter self-expanding stents, e.g Maris Deep (Invatec-Medtronic Frauenfeld, Switzerland), X-pert (Abbott Vascular, Illinois, USA) and longer-length balloon expandable stents (e.g. Chromis Deep, Invatec-Medtronic Frauenfeld, Switzerland, not FDA approved). There is no level 1 evidence to support primary bare-metal stenting over angioplasty. BMS placement has therefore been recommended to be reserved for suboptimal results after angioplasty such as residual stenosis or flow limiting dissection.  Drug-eluting stents (DESs)
Drug-eluting stents (DESs)

