Categorised under:
Interventional Radiology
>
Vascular
>
aortic stent grafts
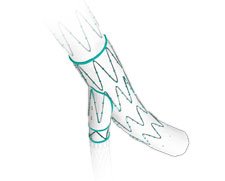
The Cook Zenith Iliac Branch Device (IBD) expands the endovascular treatment options for patients with aorto-iliac or iliac aneurysms by preserving blood flow to one or both internal iliac(IIA)arteries. This reduces potential ischaemic complications associated with occlusion of the IIAs including buttock claudication, bowel and spinal ischaemia. The IBDs are available in both standard and helical branch configurations and have previously been only available as custom devices.
The Cook Zenith Iliac Branch Device (IBD) expands the endovascular treatment options for patients with aorto-iliac or iliac aneurysms by preserving blood flow to one or both internal iliac(IIA) arteries. This reduces potential ischaemic complications associated with occlusion of the IIAs including buttock claudication , bowel and spinal ischaemia.The IBDs are available in both standard and helical branch configurations and have previously been only available as custom devices.
The iliac bifurcation device(IBD) is a modular graft component that is used in conjunction with the standard Zenith graft to treat aorto-iliac aneurysms with no common iliac landing zones. An indwelling catheter through the side branch (fig 1) allows a tru+ tru wire to be placed and a bridging stent usually a covered balloon expandable stent is placed over the aortic bifurcation to bridge between the iliac side branch and the internal iliac artery. Standard Zenith components are then placed to complete the aorto-iliac stent graft.
1) Graft planning- More complex than standard graft planning. The IBD comes in a range of common +external iliac lengths and diameters. Careful evaluation of the aortic bifurcation angle and internal iliac anatomy is needed.
2) Graft deployment - Once a tru+tru wire has been snared through the indwelling catheter(fig 2) a 10F sheath has to be passed from the contralateral groin into the opened proximal IBD- this may be difficult. We have found it best to use a co-axial sheath system and place an 8F sheath into the side branch (fig 3) . Early release of the tru +tru wire then facilitates catheterisation and tracking of the inner sheath in to the internal iliac artery. A balloon expandable covered bridging stent 9-10 mm is then placed (fig 4). We usually use the Atrium Advanta V12 for the straight branch design or the Bard Fluency for the helical branch design.
The remainder of the Zenith graft is placed via the contra lateral groin (Fig 5) and a iliac leg (stock a range of lengths) is use to bridge the gap between the short leg of the zenith body and the IBD.
We have inserted 7 of these so far, 6 of the straight branch design and one helical. We have had 100% technical success with no late complications or mortality to date.
Pros - In favourable anatomy the system works well providing an internal iliac artery preserving endovascular option for patients with aorto iliac aneurysms with no common iliac landing zones. Good training for thoraco-abdominal branch grafts.
Cons - Additional cost. Patient suitability can be limited by iliac tortuosity and large internal iliac sizes. Clinical use is limited as most cases with common iliac artery aneurysms have marked iliac tortuosity and internal iliac dilatation.
Dr John Hardman
Consultant Vascular Radiologist
UK
No conflict declared.

Did you know you can Register for FREE with this website?
Registration gives you full access to all of the features of WhichMedicalDevice. Find out more ...
WhichMedicalDevice is a FREE resource created by clinicians for clinicians.
Registration is free and gives you unlimited access to all of the content and features of this website.
Find out more...Registration is free and gives you unlimited access to all of the content and features of Which Medical Device. Find out more...
Which Medical Device is a community of clinicians sharing knowledge and experience of the devices and procedures we use on a daily basis. We ask that our members register with us so that we can maintain the unbiased and independent nature of our content. Registration is quick and free.
We do not make your details available to any third parties nor do we send unsolicited emails to our members. You can read our Privacy Policy here.