Categorised under:
Interventional Radiology
>
Vascular
>
aortic stent grafts
From Which Medical Device
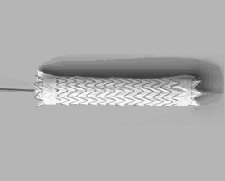
The GORE TAG Thoracic Endoprosthesis is intended for endovascular repair of aneurysms of the descending thoracic aorta in patients who have appropriate anatomy including adequate iliac/femoral access, aortic inner diameter in the range of 23-37 mm and 22 cm non-aneurysmal aorta proximal and distal to the aneurysm. This device received FDA approval in January 2012.
References
Your opinion matters to others - rate this device or add a comment

Did you know you can Register for FREE with this website?
Registration gives you full access to all of the features of WhichMedicalDevice. Find out more ...
WhichMedicalDevice is a FREE resource created by clinicians for clinicians.
Registration is free and gives you unlimited access to all of the content and features of this website.
Find out more...Registration is free and gives you unlimited access to all of the content and features of Which Medical Device. Find out more...
Which Medical Device is a community of clinicians sharing knowledge and experience of the devices and procedures we use on a daily basis. We ask that our members register with us so that we can maintain the unbiased and independent nature of our content. Registration is quick and free.
We do not make your details available to any third parties nor do we send unsolicited emails to our members. You can read our Privacy Policy here.