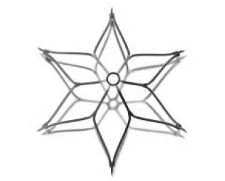
There has been a large increase in the number of caval filters inserted each year in the US, from 49,000 in 1999 to 140,000 in 2008. It is estimated that 259,000 will be inserted in 2012. This growth has been driven by rapid evolution of filter design over the last decade. However, the principle of preventing lower limb venous thrombi embolizing to the pulmonary arteries by interrupting blood flow in the inferior vena cava was established in the 19th century. Open surgical placement of a filter was first carried out in 1967 and the first percutaneous insertion of a filter (the Greenfield filter) took place in 1984.
Filters may be divided into four groups. Permanent filters such as the Greenfield filter cannot be removed. Temporary filters must be removed; these are generally tethered to the skin. Convertible filters are permanent filters that can be altered structurally after implantation to no longer function as filters; this usually involves removal of a central portion with some of the device remaining in the IVC. Optional filters may be removed or left in situ permanently. This last group has been the focus of most recent industry developments. The ideal filter is easily deployed via a small puncture site, maintains caval patency, does not perforate the cava, is biocompatible, non-thrombogenic, MR compatible and resistant to fracturing or migration. Optional filters should be easily retrievable with a long retrieval period.
This is a newer design that is based on the Günther Tulip filter and designed to be more easily retrievable. It is made of Conichrome® which is a cobalt- chromium-nickel-molybdenum-iron alloy. It has a single-cone design and incorporates secondary struts, designed to centre the filter at deployment and throughout its duration of use. It may be used in cavas up to 30mm in diameter and requires a 7F (inner diameter) sheath for insertion via the jugular route. A 2009 study found a 95% technical success rate for retrieval with a mean interval after insertion of 179 days; the longest interval was 466 days.
FDA approved for permanent use in 2007 and for retrievable use in 2008.
CE marked.
The predecessor to the Celect, it was approved for use in Europe in 1992. It consists of 4 legs, each 44mm in length, and has 12 filter wires in total. It is also made of Conichrome® and has similar insertion requirements to the Celect. No limit on the retrieval window has been set. A retrieval success rate of 94% at 12 weeks was identified in a recent study.
FDA approved for permanent use in 2000 and for retrievable use in 2003.
G2 (Bard Peripheral Vascular, Tempe, AZ, USA)
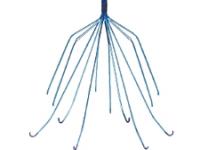
This nitinol (nickel-titanium alloy) filter has a conical shape with 6 legs and 6 filter wires arranged in 2 offset layers. It has elastic fixation hooks designed to allow easier removal. Maximum caval diameter for insertion is 28mm and it requires a 7F sheath. One study found a 95% retrieval success rate, with median indwell time of 144 days and the longest indwell time 300 days. It is an updated version of the Recovery filter, which was taken off the market in 2005 due to problems with fracturing and migration, and incorporates thicker fixation hooks and a wider leg span. However, a 2010 study found a 12% fracture rate of the G2 filter. High rates of tilting and migration have also been identified. It is likely that complications such as these prompted the FDA to issue a safety alert last year regarding adverse events in retrievable filters in general. It is retrieved using the proprietary Recovery Cone® Removal System. A variant, the G2X, has an apical hook allowing for retrieval using an endovascular snare. The Eclipse is a further variant of the G2X with a smooth electropolished surface.
FDA approved for permanent use in 2005 and for retrievable use in 2008.
OptEase (Cordis, Miami Lakes, FL, USA)

This nitinol hypotube filter is a modified version of the permanent TrapEase filter. It has a double-basket design for dual-level filtration and has side struts with unidirectional fixation barbs to protect against cephalad migration. It can be inserted via jugular, femoral or antecubital approaches but has a hook at the caudal end only and therefore may be retrieved only by a femoral approach. However this can be useful where the jugular veins are thrombosed. Maximum caval diameter for insertion is 30mm. It requires a relatively small 6F introducer system. A 2009 study found a retrieval success rate of 93% but with a mean interval of 11 days. Some studies have suggested a higher rate of IVC thrombosis due to the double-basket design; overall rates range from 0 to 12.5%. The retrieval window as indicated by the manufacturer is relatively short at 23 days.
FDA approved for permanent use in 2002 and retrievable use in 2004.
CE marked.
ALN filter (ALN Implants Chirurgicaux, Ghisonaccia, France)

This filter is made from 316L stainless steel alloy that is nonferromagnetic and is MR compatible. It has a dual level arrangement with 6 short hooked legs to ensure fixation to the caval wall and 3 longer legs for centering. It requires a 7F system for insertion. It is approved for insertion in cavas of up to 28mm diameter in the US and 32mm diameter in Europe. There is no recommendation from the manufacturer regarding retrieval window; the longest documented retrieval interval is 25 months. A 2008 study recorded a retrieval success rate of 99% after a mean interval of 93 days.
FDA approved for permanent and retrievable use in 2008. CE marked.
SafeFlo (Rafael Medical Technologies, Caesarea, Israel)
This nitinol filter has a non-tilt spiral shape, designed to be self-centering. It is anchored by an oversized double ring platform rather than by hooks or struts. This requires that the filter be sized to the caval diameter. Three sizes are available: small for cavas of 16 to 19mm, medium for 19 to 22mm and large for 22 to 25mm. It is inserted via a 6F system. The lack of hooks is thought to allow for an extended retrieval window.
FDA approved for permanent use only in 2009. CE marked.
Option (Rex Medical, distributed by Argon Medical Devices)
This is a newer design, laser cut from a single piece of nitinol for greater strength. It has a conical shape with 6 struts and a caudal hook. It is inserted via a 5F system, the smallest on the market. Maximum caval diameter is 32mm. A 2010 study recorded a retrieval success rate of 92% with mean interval of 67 days. The maximum recommended retrieval window is 175 days.
This has a novel design with two nitinol spiral elements crimped at the ends to form a symmetric double-looped helical structure. A filter mesh designed to capture clots is attached to one loop. Three fixation anchors are crimped to the opposite loop, two of which are located at the loop midpoints and the third at the tail end. Each end has a retrieval element for capture by a snare via jugular or femoral access. The symmetrical design is intended to self-centre the filter.
It is delivered via a 6F system. Two sizes are available – small for caval diameters 17 to 22mm and large for diameters 22 to 28mm.
Currently undergoing clinical trials including one FDA approved IDE trial.
Current Permanent Filters
This was the first nitinol filter on the market. It has a similar design to the G2 with a lower level of anchoring legs and an upper level for capturing clots. The main difference is that the upper level is composed of loops of wire rather than struts. Nitinol is flexible at room temperature but has thermal memory, reverting to its preformed shape at body temperature. This flexibility allows for delivery via external jugular and antecubital veins as well as the larger veins typically used. It has a good record with low rates of recurrent PE and few major complications.
FDA approved in 1990. CE mark obtained in 1998.
Vena Tech LGM (B. Braun, Melsungen, Germany)
This is a conical filter with 6 legs, each attached to a flat side rail that lies against the cava wall. These act as vertical stabilizing struts. It is made of Phynox® wire (same alloy as Conichrome®). It is delivered via a 10F system and is suitable for caval diameters up to 28mm. Some problems with incomplete opening affecting clot capturing have been reported. Filter thrombosis rates of up to 37% have been reported with consequent reduced IVC patency.
FDA approved in 1989. CE marked.
This is a newer design delivered via a much smaller 7F system. It is approved by the FDA for caval diameters up to 28mm, and CE marked for diameters up to 35mm.
FDA approved in 2001. CE marked.

This is a conical filter descended from the original Kimray-Greenfield filter. It has 6 struts, each with a curved hook. It is made of beta III titanium alloy with elastic properties but still requires a relatively large 12F system for insertion. Initial studies identified an unacceptable 30% rate of tilting, penetration and migration. This led to a modification of the hooks to reduce penetration. There are good long term patency rates with low rates of thrombosis. The Greenfield designs are regarded as the standard IVC filters because of their long track record.
FDA approved in 1989. CE marked.
This is designed to reduce the tilting problems identified with the Titanium Greenfield model by using over-the-wire insertion. It also uses an alternating hook arrangement that has been shownto reduce tilt and migration. One disadvantage is some susceptibility artifact on MR scanning.
FDA approved in 1995. CE marked.
Gianturco-Roehm Bird’s Nest (Cook Medical, Bloomington, IN, USA)
This has a unique design with two V-shaped struts supporting a random tangle of very fine wires. This is the only filter that can be used in megacavas (up to 40mm diameter); this is made possible by the 60mm maximum span of the struts. Placement is technically more complex than most filters. The wires may prolapse on insertion and for this reason it should not be placed in a suprarenal location to avoid prolapse into the heart. It requires a 12 F delivery system. It is made of biocompatible 304L stainless steel which results in marked MR susceptibility artifact.
FDA approved in 1982. CE marked.
This has a similar design to the OptEase described above. One difference is the provision of proximal and distal hooks designed to prevent migration in either the caudal or cephalad directions. It has a low rate of filter thrombosis.
FDA approved in 2000.
Discussion
Optional filters have become the first choice for most interventional radiologists due to the problems of caval thrombosis and increased DVT rate associated with permanent filters. However SIR guidelines emphasize that there are no new unique indications for optional filters as distinct from permanent filters. There remains a paucity of RCTs of filters in general with a number of unanswered questions concerning the validity of indications for filter insertion, impact of filters on mortality from PE, risk vs benefit of filter insertion, and long term outcomes. Future technological advances will include bioabsorbable convertible filters and drug-eluting filters aiming to reduce endothelialization of components.
Compare the range of currently available IVC filters mentioned in this editorial here


 This nitinol (nickel-titanium alloy) filter has a conical shape with 6 legs and 6 filter wires arranged in 2 offset layers. It has elastic fixation hooks designed to allow easier removal. Maximum caval diameter for insertion is 28mm and it requires a 7F sheath. One study found a 95% retrieval success rate, with median indwell time of 144 days and the longest indwell time 300 days. It is an updated version of the Recovery filter, which was taken off the market in 2005 due to problems with fracturing and migration, and incorporates thicker fixation hooks and a wider leg span. However, a 2010 study found a 12% fracture rate of the G2 filter. High rates of tilting and migration have also been identified. It is likely that complications such as these prompted the FDA to issue a safety alert last year regarding adverse events in retrievable filters in general. It is retrieved using the proprietary Recovery Cone® Removal System. A variant, the G2X, has an apical hook allowing for retrieval using an endovascular snare. The Eclipse is a further variant of the G2X with a smooth electropolished surface.
This nitinol (nickel-titanium alloy) filter has a conical shape with 6 legs and 6 filter wires arranged in 2 offset layers. It has elastic fixation hooks designed to allow easier removal. Maximum caval diameter for insertion is 28mm and it requires a 7F sheath. One study found a 95% retrieval success rate, with median indwell time of 144 days and the longest indwell time 300 days. It is an updated version of the Recovery filter, which was taken off the market in 2005 due to problems with fracturing and migration, and incorporates thicker fixation hooks and a wider leg span. However, a 2010 study found a 12% fracture rate of the G2 filter. High rates of tilting and migration have also been identified. It is likely that complications such as these prompted the FDA to issue a safety alert last year regarding adverse events in retrievable filters in general. It is retrieved using the proprietary Recovery Cone® Removal System. A variant, the G2X, has an apical hook allowing for retrieval using an endovascular snare. The Eclipse is a further variant of the G2X with a smooth electropolished surface.  This nitinol hypotube filter is a modified version of the permanent TrapEase filter. It has a double-basket design for dual-level filtration and has side struts with unidirectional fixation barbs to protect against cephalad migration. It can be inserted via jugular, femoral or antecubital approaches but has a hook at the caudal end only and therefore may be retrieved only by a femoral approach. However this can be useful where the jugular veins are thrombosed. Maximum caval diameter for insertion is 30mm. It requires a relatively small 6F introducer system. A 2009 study found a retrieval success rate of 93% but with a mean interval of 11 days. Some studies have suggested a higher rate of IVC thrombosis due to the double-basket design; overall rates range from 0 to 12.5%. The retrieval window as indicated by the manufacturer is relatively short at 23 days.
This nitinol hypotube filter is a modified version of the permanent TrapEase filter. It has a double-basket design for dual-level filtration and has side struts with unidirectional fixation barbs to protect against cephalad migration. It can be inserted via jugular, femoral or antecubital approaches but has a hook at the caudal end only and therefore may be retrieved only by a femoral approach. However this can be useful where the jugular veins are thrombosed. Maximum caval diameter for insertion is 30mm. It requires a relatively small 6F introducer system. A 2009 study found a retrieval success rate of 93% but with a mean interval of 11 days. Some studies have suggested a higher rate of IVC thrombosis due to the double-basket design; overall rates range from 0 to 12.5%. The retrieval window as indicated by the manufacturer is relatively short at 23 days. This filter is made from 316L stainless steel alloy that is nonferromagnetic and is MR compatible. It has a dual level arrangement with 6 short hooked legs to ensure fixation to the caval wall and 3 longer legs for centering. It requires a 7F system for insertion. It is approved for insertion in cavas of up to 28mm diameter in the US and 32mm diameter in Europe. There is no recommendation from the manufacturer regarding retrieval window; the longest documented retrieval interval is 25 months. A 2008 study recorded a retrieval success rate of 99% after a mean interval of 93 days.
This filter is made from 316L stainless steel alloy that is nonferromagnetic and is MR compatible. It has a dual level arrangement with 6 short hooked legs to ensure fixation to the caval wall and 3 longer legs for centering. It requires a 7F system for insertion. It is approved for insertion in cavas of up to 28mm diameter in the US and 32mm diameter in Europe. There is no recommendation from the manufacturer regarding retrieval window; the longest documented retrieval interval is 25 months. A 2008 study recorded a retrieval success rate of 99% after a mean interval of 93 days. 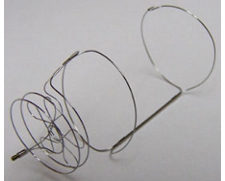
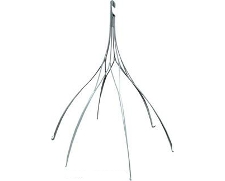

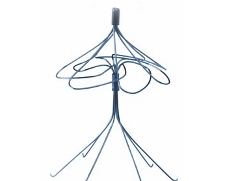


 This is a conical filter descended from the original Kimray-Greenfield filter. It has 6 struts, each with a curved hook. It is made of beta III titanium alloy with elastic properties but still requires a relatively large 12F system for insertion. Initial studies identified an unacceptable 30% rate of tilting, penetration and migration. This led to a modification of the hooks to reduce penetration. There are good long term patency rates with low rates of thrombosis. The Greenfield designs are regarded as the standard IVC filters because of their long track record.
This is a conical filter descended from the original Kimray-Greenfield filter. It has 6 struts, each with a curved hook. It is made of beta III titanium alloy with elastic properties but still requires a relatively large 12F system for insertion. Initial studies identified an unacceptable 30% rate of tilting, penetration and migration. This led to a modification of the hooks to reduce penetration. There are good long term patency rates with low rates of thrombosis. The Greenfield designs are regarded as the standard IVC filters because of their long track record.