Gore receives FDA approval for TAG Thoracic Endoprosthesis
Published date : 19 January 2012
Article date : 19 January 2012
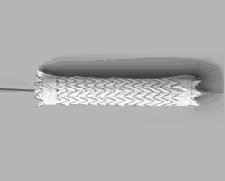 The FDA has approved the GORE TAG Thoracic Endoprosthesis for endovascular repair of the descending thoracic aorta. The device is the first endovascular grafting system approved by the FDA to treat aneurysms of the thoracic aorta.
The FDA has approved the GORE TAG Thoracic Endoprosthesis for endovascular repair of the descending thoracic aorta. The device is the first endovascular grafting system approved by the FDA to treat aneurysms of the thoracic aorta.
The full approval letter can be found here.
Find out more about the GORE TAG Thoracic Endoprosthesis here.


