Categorised under:
Interventional Radiology
>
Vascular
>
aortic stent grafts
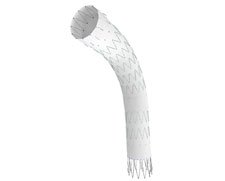
The TX2 is an endovascular stent graft for treatment of thoracic aortic diseases which replaces the original TX1 graft. This is of similar construction and delivery to the highly proven and successful Zenith abdominal aortic stent graft. In December 2006 the TX delivery system was further enhanced with the introduction of the Z-Trak Plus system. The stent graft is constructed of stainless steel Z stents sutured on to a polyester fabric graft. Fixation to the aortic wall is enhanced by circumferentially orientated anchoring barbs both proximally and distally. The graft is mounted within a hydrophilic braided sheath with a super-elastic alloy inner cannula and is available as a single or two piece device. The 2 piece device is available in 28-42mm diameters and in both straight and 4 mm tapered components with lengths ranging from 120 -220mm. A trigger wire delivery mechanism allows partial deployment endograft in a trifold configuration.
The TX2™ is an endovascular stent graft for treatment of thoracic aortic diseases which replaces the original TX1 graft. This is of similar construction and delivery to the highly proven and successful Zenith abdominal aortic stent graft. In December 2006 the TX2™ delivery system was further enhanced with the introduction of the Z-Trak Plus system.
The stent graft is constructed of stainless steel Z stents sutured on to a polyester fabric graft. Fixation to the aortic wall is enhanced by circumferentially orientated anchoring barbs both proximally and distally .The graft is mounted within a hydrophilic braided sheath with a super-elastic alloy inner cannula and is available as a single or two piece device. The 2 piece device is available in 28-42mm diameters and in both straight and 4 mm tapered components with lengths ranging from 120 -220mm. A trigger wire delivery mechanism allows partial deployment endograft in a trifold configuration.
The delivery system has a low profile sheath with 20 French diameters for up to 34mm grafts and 22 French for the larger systems. This is combination with the hydrophilic coating facilitates access through small and tortuous iliac vessels. Track ability of the system from there up and round the arch is impressive because of the combination the kink resistant braided flexor sheath and the super elastic alloy inner cannula. This ensures a good balance of graft push ability and flexibility . Once the graft is opened the trigger wire delivery mechanism allows further fine adjustment of the endograft in a semi deployed state. The trigger wires hold the graft in trifold configuration allowing blood to flow around the outside of the graft reducing the wind sock affect which can result in displacement of the graft backwards. At this stage further fine adjustment is possible before the trigger wires are released and the graft is fully deployed.
Experience of using the TX2 graft since Dec 2004 is that the graft delivery system is intuitive and that user confidence is high because the control is maintained through out each stage of deployment. On the negative side proximal barbs may be traumatic in patients with aortic dissection. If this is a concern the graft can be manufactured without them.
New innovations on the TX2 thoracic stent graft with the Z-Trak Plus introduction system has enhanced the delivery, control and precision of placement of the TX2 thoracic endograft.
Dr John Hardman
Consultant Vascular Radiologist, UK
No conflict declared.

Did you know you can Register for FREE with this website?
Registration gives you full access to all of the features of WhichMedicalDevice. Find out more ...
WhichMedicalDevice is a FREE resource created by clinicians for clinicians.
Registration is free and gives you unlimited access to all of the content and features of this website.
Find out more...Registration is free and gives you unlimited access to all of the content and features of Which Medical Device. Find out more...
Which Medical Device is a community of clinicians sharing knowledge and experience of the devices and procedures we use on a daily basis. We ask that our members register with us so that we can maintain the unbiased and independent nature of our content. Registration is quick and free.
We do not make your details available to any third parties nor do we send unsolicited emails to our members. You can read our Privacy Policy here.