Categorised under:
Interventional Radiology
>
Biopsy and Drainage
>
Biopsy guns and needles
The Spirotome has 4 parts: the TROCAR needle, the RECEIVING needle, the CUTTING CANNULA and the release element. The device is designed to take high quality large core soft tissue biopsies. Cores of 20mm long and 3mm diameter can be harvested without discomfort to the patient.
The Spirotome has 4 parts: the TROCAR needle, the RECEIVING needle, the CUTTING CANNULA and the release element. The device is designed to take high quality large core soft tissue biopsies. Cores of 20mm long and 3mm diameter can be harvested without discomfort to the patient.
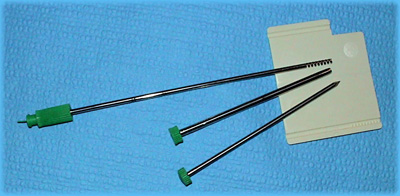
The device has been designed to take tissue samples for histological and laboratory analysis, for example in the investigation of suspected of breast cancer. Following skin disinfection, local anesthesia of the skin and subdermal layers is performed up to the maximum depth of biopsy. Then a small skin incision is made to facilitate penetration of the biopsy instrument. The trocar and cutting cannula are mounted and advanced to the lesion for biopsy, and the exact position can be confirmed by ultrasound or stereotactic mammography. Once correct position is confirmed, the trocar is replaced by the receiving needle.
The helix can now penetrate the lesion by twisting (this can be visualized when using ultrasound guidance) up to a maximum of 2 cm. The position of the helix in the lesion can be documented by imaging. The receiving needle is now fixed and the cutting cannula rotated and advanced over the helix, cutting through the tissue and separating the sample.
Once the first mark on the receiving needle is seen, the helix and sample are completely in the cutting cannula and the receiving needle can be withdrawn. The tissue can be released through the release element and immersed in the fixator. The procedure can be repeated without removing the cutting cannula, which can be repositioned within the limits imposed by skin flexibility at the puncture site (similar to Vacuum Assisted biopsy (VAB) devices.
Combined results from prospective safety and efficacy clinical trials (n=800)
Representativity:
Patient comfort
Costs
Access
The Spirotome (a direct and frontal – D&F type of instrument) has been compared to surgery in relation to sample quality to be used for quantitative molecular biology. The comparison with regard to representativity of the sample, comfort for the patient, costs and access (dedicated application rooms) is shown below for Tru-cut systems, Vacuum Assisted Biopsy tools and open surgery.
| Tru-cut | D & F | VAB | Surgery | |
| Representivity | No | Yes | Yes | Yes |
| Comfort | Yes | Yes | No | No |
| Costs | Yes | Yes | No | No |
| Access | Yes | Yes | No | Yes |
This device is simple to use, quicker than most VAB devices, cheaper and more acceptable to patients. Positioning is very accurate both with ultrasound and stereotactic mammographic guidance and the positive yield for histology as very high.
Dr Richard Harries, Consultant Radiologist
Diana, Princess of Wales Hospital
No conflict declared
1. Luc Rotenberget al.Multicentre clinical experience with large core soft tissue biopsy without vacuum assistance Eur J Cancer Prev. 13(6): 491-8, 2004.
2. Wojciech P Polkowski. Spirotome as an alternative to vacuum assisted mammotome biopsy systems.Pol J Radiol 72: 43 – 47, 2007
3. Ann Cornelis et al. Efficacy and safety of direct and frontal macrobiopsies in breast cancer. Eur J Cancer Prev2009 ; 18 : 280 – 4.
4. Harries R, Lawson S, Bruckers Liesbeth. Accuracy of Direct and Frontal Biopsy System in the assessment of microcalcifications of the breast. Eur J Cancer Prev 2010; 18. In Press

Did you know you can Register for FREE with this website?
Registration gives you full access to all of the features of WhichMedicalDevice. Find out more ...
WhichMedicalDevice is a FREE resource created by clinicians for clinicians.
Registration is free and gives you unlimited access to all of the content and features of this website.
Find out more...Registration is free and gives you unlimited access to all of the content and features of Which Medical Device. Find out more...
Which Medical Device is a community of clinicians sharing knowledge and experience of the devices and procedures we use on a daily basis. We ask that our members register with us so that we can maintain the unbiased and independent nature of our content. Registration is quick and free.
We do not make your details available to any third parties nor do we send unsolicited emails to our members. You can read our Privacy Policy here.