Categorised under:
Interventional Radiology
>
Vascular
>
Embolic Liquids
Tissue adhesives based on thrombin have been used in surgery since the 1940s. They are commonly used in neurosurgery (CSF leaks), vascular surgery and for haemostasis during tumour resections. The first use of thrombin to occlude aneurysms and pseudoaneurysms was reported in the AJR in 1986 by Cope and Zeit. They percutaneously injected thrombin into iliac, peroneal and hepatic artery pseudoaneurysms successfully occluding them. Direct injection of thrombin has become popular again since the late 1990s for the treatment of iatrogenic femoral pseudoaneurysms (refs). There are several preparations on the market. The one we use in our centre is Tisseel.
Tissue adhesives based on thrombin have been used in surgery since the 1940s. They are commonly used in neurosurgery (CSF leaks), vascular surgery and for haemostasis during tumour resections. The first use of thrombin to occlude aneurysms and pseudoaneurysms was reported in the AJR in 1986 by Cope and Zeit. They percutaneously injected thrombin into iliac, peroneal and hepatic artery pseudoaneurysms successfully occluding them. Direct injection of thrombin has become popular again since the late 1990s for the treatment of iatrogenic femoral pseudoaneurysms (refs). There are several preparations on the market. The one we use in our centre is Tisseel.
Tisseel is a prescription only product and is licensed for supportive treatment to aid standard surgical techniques to improve haemostasis and as a tissue glue eg for GI anastamoses. Tisseel mimics the final stages of the coagulation cascade using high concentrations of fibrin and thrombin. It is contraindicated intravascularly and in patients with hypersensitivity to any components.
Component 1:
The sealer Protein Solution containing
Human Fibrinogen (Clottable Protein) 91 mg/ml
Aprotinin 3000 KIU/ml
Component 2:
The thrombin solution containing
Human Thrombin 500 IU/ml
Calcium Chloride 40 μmol/ml
The thrombin and fibrinogen are derived from pooled human plasma and are double treated for viral inactivation. The Aprotinin (an antifibrinolytic) is derived from bovine plasma, but note that this component is not used for pseudoaneurysm ablation.
The only components of the kit needed are the thrombin and calcium chloride solution. (PIC) The thrombin acts on fibrinogen within the blood converting it rapidly to fibrin which crosslinks to form the basis of the thrombus.
Femoral pseudo aneurysm ablation is a simple procedure performed under ultrasound guidance.
1. The calcium chloride solution is drawn up into a syringe.
2. This is injected into the bottle containing the dried thrombin powder. This is slowly swirled to dissolve (not shaken). In theory the two vials should be at 37 degrees, but room temperature appears to be adequate.
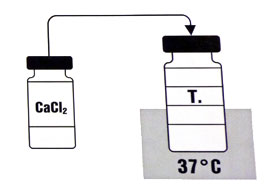
3. The solution is drawn back into a syringe.
4. The pseudoaneurysm is then percutaneously punctured with usually an 18g needle. It is best if the needle tip is kept away from the neck of the pseudoaneurysm due to the risk of inadvertent intra arterial injection.
5. The thrombin solution is then slowly injected into the cavity causing almost instantaneous thrombosis. This often occurs after less than 0.5ml is injected.
The full procedure is illustrated in two of our videos:
Complications are usually due to hypersensitivity reactions and distal embolisation due to thrombin escaping into the peripheral circulation. In over 150 cases we have never had a case of hypersensitivity or significant distal embolisation. Small emboli will usually lyse spontaneously. In the case of significant distal embolisation I would suggest clot aspiration and/or small amounts of local thrombolytic. Tisseel is of course not licensed for intravascular use and it could be debated that this is 'off-label' device use. Is a pseudoaneurysm intravascular?
A simple technique to occlude femoral and other pseudoaneurysms. Complications are minimal and it has proven to be far superior to femoral artery compression which is now almost unheard of.

Did you know you can Register for FREE with this website?
Registration gives you full access to all of the features of WhichMedicalDevice. Find out more ...
WhichMedicalDevice is a FREE resource created by clinicians for clinicians.
Registration is free and gives you unlimited access to all of the content and features of this website.
Find out more...Registration is free and gives you unlimited access to all of the content and features of Which Medical Device. Find out more...
Which Medical Device is a community of clinicians sharing knowledge and experience of the devices and procedures we use on a daily basis. We ask that our members register with us so that we can maintain the unbiased and independent nature of our content. Registration is quick and free.
We do not make your details available to any third parties nor do we send unsolicited emails to our members. You can read our Privacy Policy here.